Endogenex is a company pioneering a treatment for type 2 diabetes that targets what research now suggests is a likely driver of the disease: a damaged duodenum. The company’s novel, outpatient endoscopic procedure uses non-thermal pulsed electric fields (PEF) energy to treat inflamed and dysfunctional tissue associated with type 2 diabetic duodenopathy. Pulse 2.0 interviewed Endogenex’s VP of R&D and Operations, Matt Cambronne, to gain a deeper understanding of the company.
Matt Cambronne’s Background

What is Matt Cambronne’s background? Cambronne said:
“I’ve been drawn to working in medical technology since the early 2000s. After studying Materials Science and Engineering at Iowa State University and earning a business degree at Augsburg College, I started my career at Boston Scientific, where I was fortunate to contribute to the development of multiple novel products within the cardiovascular space.”
“After five years, I wanted to experience life at a smaller company, which led me to join Cardiovascular Systems, Inc. (CSI), then a 40-person clinical-stage startup. Over time, I grew to lead the R&D team, helping to scale CSI into an 850-person market leader, ultimately acquired by Abbott in 2023. I helped to lead the diligence, acquisition and integration – an incredible experience – but soon felt pulled back to the startup world. I joined Endogenex in late 2023, excited by its strong team, technology and potential impact.”
Formation Of The Company
How did the idea for the company come together? Cambronne shared:
“Endogenex was born out of an interesting clinical insight: That when people with type 2 diabetes (T2D) underwent gastric bypass surgery – where the digestive tract is rerouted to bypass the duodenum, the top part of the small intestine – their T2D was disrupted, with about half achieving remission within two to three weeks post-surgery.”
“Dr. Barham Abu Dayyeh – then at Mayo Clinic – became fascinated with exploring this phenomenon. Along with other contributors, they began to investigate the role of the duodenum in metabolic regulation. They conceived the idea to use pulsed electric field energy to treat the duodenum, with the hypothesis that it would impact type 2 diabetes and weight loss. Their research led to the development of a technology platform that was spun out from Mayo Clinic as Endogenex.”
Core Products
What are the company’s core products and features? Cambronne explained:
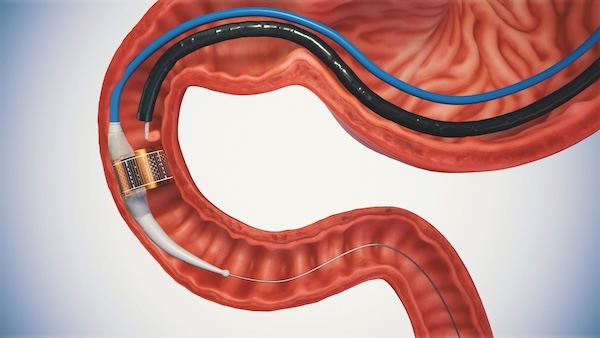
“Endogenex has developed a novel, outpatient endoscopic procedure that uses non-thermal pulsed electric field (PEF) energy to treat inflamed and dysfunctional tissue in the duodenum – the top part of the small intestine, where metabolic coordination occurs – in people with type 2 diabetes. The goal of the Endogenex treatment is to eliminate the unhealthy duodenal tissue and restore the gut to a healthier state to potentially slow or stop the diabetes disease progression.”
“The Endogenex system consists of an electrical pulse generator as well as a specialized catheter that delivers PEF to two layers of the tissue in the duodenum – the mucosa and sub-mucosa – to initiate the body’s natural process of cell regeneration in the tissues that need it most (without damaging other tissues).”
“The Endogenex procedure is a minimally invasive, same-day procedure, and nothing is left behind in the patient.”
Evolution Of The Company’s Technology
How has the company’s technology evolved since its inception? Cambronne noted:
“Now three generations in, we have evolved our catheter technology to improve both efficacy and ease of use. Some of the unique catheter design features include:
— The flexible electrode circuit that is engineered to work on a broad range of duodenum sizes by using a rolled ‘film canister’ design – allowing it to expand or contract as necessary to achieve full contact with the duodenal wall.
— Added design features – such as ‘wings’ and a suction extension accessory – to prevent the trapping of mucosal tissue in and around the flex circuit and to help ensure there is optimal contact between the flexible electrode circuit and the duodenal wall to maximize treatment effect.
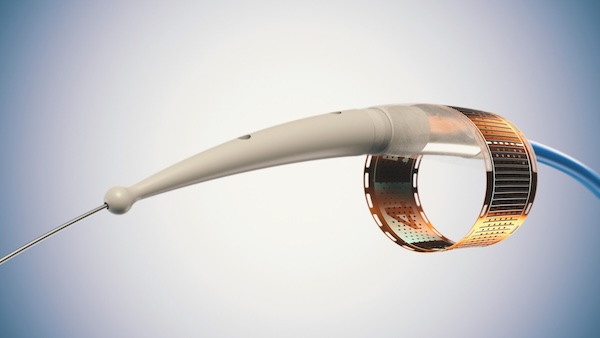
Similarly, the electrical pulse generator technology has been improved to optimize voltage output, including having automatic voltage and current monitoring during therapy delivery; and to reduce the total time at each treatment site (from approximately 8 minutes to 1 minute). We also figured out how to go from 30 cabling connections for the generator, down to 1 – ensuring MUCH easier use for healthcare providers.
Together, these technology enhancements have resulted in increased procedural efficiency and a defined long-term treatment effect based on our early clinical experience.”
Significant Milestones
What have been some of the company’s most significant milestones? Cambronne cited:
“Endogenex has seen some exciting milestones, including:
— Receiving FDA Breakthrough Device designation – an expedited FDA review process for novel medical devices ‘that have the potential to provide more effective treatment or diagnosis for life-threatening or irreversibly debilitating diseases or conditions’ – in May 2022
— Achieving successful enrollment and completing our REGENT-1 feasibility studies in the U.S. and Australia, recruiting more than 70 people with T2D who were interested in helping us understand the feasibility and preliminary safety of the Endogenex device
— Receiving Investigational Device Exemption (IDE) approval from the FDA to initiate our ReCET clinical study of the Endogenex procedure for T2D patients – a critical step in advancing the procedure and continuing our R&D journey.”
Favorite Memory
What has been your favorite memory working for the company so far? Cambronne reflected:
“My favorite memory so far happened when we received FDA approval to use the third generation of our catheter and generator technology in the ReCET pivotal clinical study. The initiation of patient enrollments in the study was gated to the qualification and approval of this third generation of the system, which meant delivering with both quality and speed was of the utmost importance. In the end, the combined Endogenex team worked together to deliver a product and submission that received rapid FDA review and approval. The degree to which the Endogenex team has been able to iterate and improve the technology over the course of just a few years is remarkable given the system’s complexity.”
Customer Success Stories
When asking Cambronne about patient success stories, he highlighted:
“Patient success stories are one of the most rewarding parts of the job!
Chad was an early participant in our REGENT-1 U.S. study. He enrolled in the study at age 51 after he had been working to manage his T2D for years by eating better and taking medication, but he was still not satisfied with the results. In an effort to avoid going on insulin, he decided to join the study, met the criteria, and received the Endogenex procedure.
Four months after he received his treatment, he had lost 30 pounds, his A1C went down to 5.7 percent, and he was able to stop taking his blood pressure medication. Now, of course, not all patients have the same outcomes, and participating in the study doesn’t guarantee anything – but I was so happy to hear about his experience.”
Total Addressable Market
What total addressable market (TAM) size is the company pursuing? Cambronne assessed:
“Type 2 diabetes is a growing epidemic. Our indicated population exceeds 5 million U.S. adults. Longer term, we see broader potential – with 39 million adults in the U.S. having type 2 diabetes and over 100 million having prediabetes. We’re looking forward to seeing where the research takes us.”
Differentiation From The Competition
What differentiates the company from its competition? Cambronne affirmed:
“There are other companies targeting the duodenum for the treatment of type 2 diabetes, but Endogenex is the only one that’s using a non-thermal, pulsed electric field (PEF) energy source instead of thermal energy sources such as steam or lasers.”
“This goes back to an early discovery by Dr. Abu Dayyeh and his colleagues: that there was a significant benefit to using bipolar electrodes along with a biphasic pulse waveform for treatment. This approach allows for more precise control when delivering energy to the tissue, compared to older or more traditional techniques like radiofrequency or other heat-based treatments. As a result, Endogenex technology can safely target and destroy specific layers of cells in the lining of the gut including the mucosa and submucosa, while leaving the surrounding structure intact and reducing the risk of damage and inflammation to nearby tissue.”
“This is important because our research suggests that targeting both the mucosa and submucosa may optimize the long-term benefit to patients.”
Future Company Goals
What are some of the company’s future goals? Cambronne concluded:
“Right now, Endogenex is singularly focused on bringing our technology to market for people with T2D: through completing enrollment in our ReCET clinical study, and continuing to evolve our technology to ensure we’re meeting requirements for broad commercial scale and physician adoption.”
“The future is bright as we continue to explore how our PEF technology can be applied beyond the duodenum to address a broader range of gastrointestinal and metabolic diseases.”


