PolyPid is a biopharmaceutical company developing localized, controlled-delivery antibiotic therapies to prevent surgical site infections. Pulse 2.0 interviewed PolyPid Chief Operating Officer – U.S. Operations, Ori Warshavsky, to gain a deeper understanding of the company.
Ori Warshavsky’s Background

Could you tell me more about your background? Warshavsky said:
“I’ve always been involved in biotech and pharma, from my early days as a biotech engineering undergrad. After spending a few years working in a lab, I quickly realized that bench science was not where my real strengths lay, so I pivoted to more of a business focus by pursuing an MBA at Duke University, focusing on Health Sector Management.”
“Following my MBA, I joined Teva Pharmaceuticals, where I spent over a decade in various corporate strategy, business development, and commercial roles. This included two years as Chief of Staff to the CEO of Global Generics Medicine, which exposed me to the vast world of Teva’s operations across various markets and products. From there I relocated to Montreal, Canada to lead Teva’s market access activities, gaining tremendous experience learning how to deal with new markets in terms of access, government affairs and patient advocacy. A few years later, I returned to the U.S. to join the oncology team where, as Senior Director of Oncology Marketing, I helped launch Teva’s first two biosimilars – for Rituxan and Herceptin – at a time when biosimilars were only just emerging in the market.”
“Then came an opportunity to join PolyPid as VP Marketing. What initially drew me to the company was the chance to work for a startup developing a solution that brings clear and immediate benefit for patients. True to startup life, my role quickly expanded and I found myself tasked with leading pre-launch activity planning for marketing, sales, medical affairs, supply chain, and essentially building out the company’s U.S. operations. Today, as Chief Operating Officer, I oversee all U.S.-related activities, including business development, investor relations, and everything tied to launch efforts for our lead product, D-PLEX100.”
Formation Of The Company
How did the idea for the company come together? Warshavsky shared:
“Our founder, Dr. Noam Emanuel, completed his PhD in Immunology and Drug Delivery at the Hebrew University, under the supervision of Prof. Chezy Barenholz, whose lab produced a number of novel drugs. After completing his PhD, Dr. Emanuel moved into a different field entirely, until he received an inquiry from two dental surgeons looking for better infection prevention strategies, specifically around local antibiotic delivery in dental surgery. Expecting it would be a fairly straightforward research task, he was surprised to discover that there was no effective solution for local, prolonged antibiotic release.”
“That lit the spark and set him on the path of investigating the field of surgical site infections, which led to him entering an incubator program, securing initial funding, and eventually launching PolyPid in 2008. Since then, we’ve moved from the dental space to addressing other indications, which led to where we are today as a global pre-NDA stage biopharma company developing novel, locally administered therapies to improve surgical outcomes.”
Favorite Memory
What has been your favorite memory working for the company so far? Warshavsky reflected:
“It happens to be a very recent one. After almost 20 years in the pharma space, I thought I’d seen it all – clinical successes and failures, product launches, mergers and acquisitions. But before joining PolyPid, I’d never had the chance to witness first-hand the successful completion of a Phase 3 trial for an approvable drug. That changed in June when we announced positive results from our second Phase 3 trial. It was an incredibly meaningful moment – one that many in our industry work towards, but often don’t get to experience first-hand.”
Core Products
What are the company’s core products and features? Warshavsky explained:
“PLEX is a novel drug delivery platform for local, high concentration prolonged release of many types of Active Pharmaceutical Ingredients (APIs), capable of encapsulating and releasing anything from small molecules to peptides, proteins and nucleic acids over long periods of time.”
“Our lead product, D-PLEX100, is designed to provide local prolonged and controlled anti-bacterial activity directly at the surgical site to prevent surgical site infection (SSI). Following administration into a surgical site, the PLEX technology, paired with doxycycline, enables prolonged and continuous release of the broad-spectrum antibiotic. This enables the release of high local concentration of the drug to prevent SSIs for 30 days.”
“The SSI space has seen very little innovation for at least a decade, with the result that both surgeons and patients have come to expect infections as par for the course when undergoing surgery. We introduced D-PLEX100 to break that paradigm.”
Challenges Faced
Have you faced any challenges in your sector of work recently? Warshavsky acknowledged:
“One of our biggest challenges came during our first Phase 3 trial, SHIELD I, which we designed in 2019 and launched in July 2020. This coincided with the COVID-19 pandemic, when hospital behaviors changed dramatically – namely, elective surgeries were canceled and, due to people actively avoiding hospitals, infection rates all but disappeared. Having planned our trial in 2019, the world as we knew it changed in 2020, and our trial failed. As one colleague described it, testing an infection-prevention product in an environment where surgeries weren’t happening was akin to “testing a raincoat during a drought.”
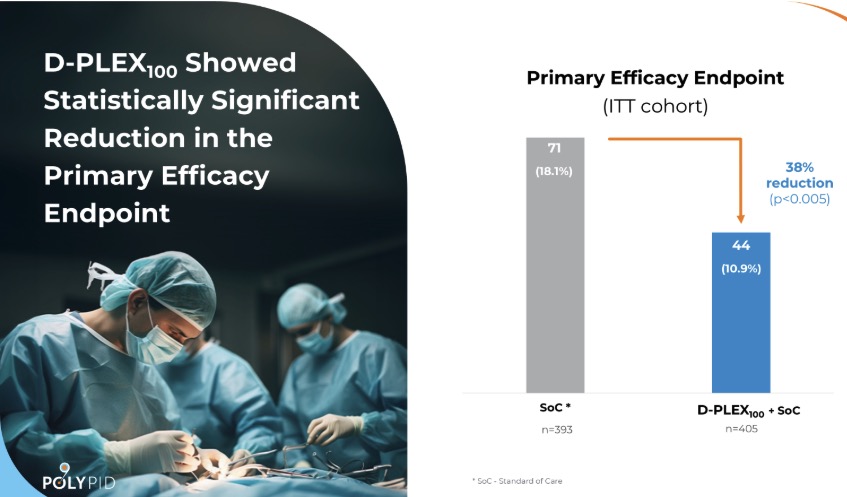
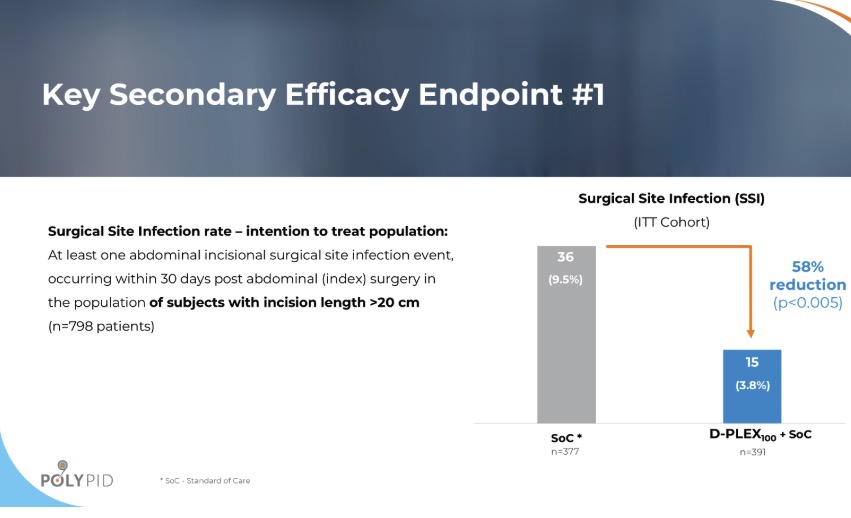
“Following that initial failure, we had to figure out what we could do to resurrect the product. This involved major restructuring and tough decisions had to be made to preserve resources and find a path forward. After three years, these efforts paid off and we were able to launch a second, more targeted trial that succeeded in meeting all the primary and key secondary endpoints.”
Evolution Of The Company’s Technology
How has the company’s technology evolved since launching? Warshavsky noted:
“The core technology and delivery system has remained the same, but we’ve adapted how and where we use it. Having started in dental surgeries, we then pivoted to focus on infections in bones and later, more specifically, sternum-related infections. Despite a clear market need, those indications required massive trials that weren’t feasible for a company of our size at the time. Thus, we pivoted again to focus on colorectal resection procedures, which is where we are now. This is an area with high infection rates, providing us with a clear pathway to demonstrate the effectiveness of our product within manageable trial sizes.”
“Beyond that, we’ve conducted intensive product tests based on the concept of local and prolonged delivery in the GLP-1 receptor agonist space. Current once-weekly injections can have serious side effects, which partially stem from the peak and trough of their release profile. Our platform enables the release of GLP-1 receptor agonists over 50 to 60 days in a linear way, which we anticipate will reduce the GI side effects and improve patient adherence over time. We are also working on prolonged delivery of oncolytic agents intratumorally to eliminate solid tumors locally without the systemic side effects of current therapies.
Significant Milestones
What have been some of the company’s most significant milestones? Warshavsky cited:
“A number of key milestones stand out, since the company’s launch. In June 2020, we completed an IPO, which coincided with the start of our first Phase 3 trial. Those two major milestones followed on the heels of a very successful Phase 2 trial, in which our technology demonstrated the ability to reduce infection by over 50%. In August 2022, we signed an exclusive licensing agreement with ADVANZ PHARMA to fully commercialize our product in Europe. Other strategic partnerships include an R&D collaboration with ImmunoGenesis, signed in December 2024, which applies our platform to enhance ImmunoGenesis’ therapeutic for treating solid tumors. Finally, in June this year, we completed our successful second Phase 3 trial, which launched in 2023.”
Revenue/Funding
Are you able to discuss funding and/or revenue metrics? Warshavsky revealed:
“Over the years, we’ve invested over $250 million into the development of D-PLEX100, including building our own manufacturing capabilities. As of today, we are fully funded through our New Drug Application (NDA) FDA submission. We also expect to receive milestone payments from our European partnership and are currently in the process of securing a U.S. commercialization deal, which would include upfront payments. So, overall, we expect to be fully funded up to and beyond the NDA approval.”
Total Addressable Market
What total addressable market (TAM) size is the company pursuing? Warshavsky assessed:
“In the U.S. alone, CDC estimates SSI to occur in 2–5% of all surgeries performed each year, resulting in estimated direct medical costs of between $3.5–10 billion. Specifically for D-PLEX100, we are targeting 12 million procedures that have either high patient burden, such as abdominal procedures where infection can lead to an additional 7-10 days in the hospital, or high financial cost like joint replacement, where infection can lead to weeks in the hospital and close to $100K of direct costs.”
Differentiation From The Competition
What differentiates the company from its competition? Warshavsky affirmed:
“Our PLEX technology has some key differentiators:
- While competing solutions typically achieve between 72 and 96 hours of drug release, our technology can release targeted therapies over a period of days to months – a completely different release duration, which can result in greater efficacy and safety profiles.
- Most delivery systems tend to cause an initial spike in drug concentration (sometimes even reaching toxic levels), followed by a drop off and then a long tail of release, which is often outside the drug’s optimal window of activity. Our proprietary delivery system ensures a consistent and linear release of therapeutics in the activity window throughout the entire treatment duration.
- While most competing products are medical devices with minimal supporting data, our lead product, D-PLEX100, is a pharmaceutical and comes with extremely robust clinical data, having conducted two Phase 3 trials, each comprising close to 1,000 patients.”
Future Company Goals
What are some of the company’s future goals? Warshavsky concluded:
“In the near term, our immediate focus is on filing for the NDA for D-PLEX100 early next year. This involves a pre-NDA meeting with the FDA before the end of 2025 and preparing for inspection of our manufacturing site ahead of the submission. We are also actively seeking a U.S. commercialization partner to launch D-PLEX100 in the States.”
“In the longer term, being a platform company, we will focus on continued development of our product pipeline. We see great potential for expanding the use of localized delivery of therapeutics in indications that currently rely on systemic exposure, which we hope to announce in future.”


